Malic acid
| Malic acid | |
|---|---|
 |
|
 |
|
|
hydroxybutanedioic acid
|
|
|
Other names
L-Malic acid
D-Malic acid (-)-Malic acid (+)-Malic acid (S)-Hydroxybutanedioic acid (R)-Hydroxybutanedioic acid |
|
| Identifiers | |
| CAS number | 6915-15-7 |
| ChemSpider | 510 |
| EC number | 230-022-8 |
| Properties | |
| Molecular formula | C4H6O5 |
| Molar mass | 134.09 g mol−1 |
| Density | 1.609 g/cm³ |
| Melting point |
130 °C, 403 K, 266 °F |
| Solubility in water | 558 g/l (at 20 °C)[1] |
| Acidity (pKa) | pKa1 = 3.4, pKa2 = 5.13 |
| Related compounds | |
| Other anions | malate |
| Related carboxylic acids | propionic acid butyric acid succinic acid tartaric acid crotonic acid fumaric acid pentanoic acid |
| Related compounds | butanol butyraldehyde crotonaldehyde sodium malate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Malic acid is an organic compound with the formula HO2CCH2CHOHCO2H. This dicarboxylic acid is the active ingredient in many sour or tart foods. Malic acid is found mostly in unripe fruits.
The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.
Contents |
History
Malic acid was first isolated from apple juice by Carl Wilhelm Scheele in 1785. Antoine Lavoisier in 1787 proposed the name acide malique which is derived from the Latin word for apple, mālum.[2]
Chemical structure
In biological sources, malic acid is homochiral. Malic acid has two stereoisomers, a left-handed L-enantiomer and a right-handed D-enantiomer. L-Malic acid is the naturally occurring form, whereas D-malic acid is produced synthetically.
 |
 |
Biochemistry
Malate plays an important role in biochemistry. In the C4 carbon fixation process, malate is a source of CO2 in the Calvin cycle. In the citric acid cycle, (S)-malate is an intermediate, formed by the addition of an -OH group on the si face of fumarate. It can also be formed from pyruvate via anaplerotic reactions.
Malate is also produced from starch in guard cells of plant leaves. A build up of malate leads to a low water potential. Water then flows into the guard cells causing the stoma to open. However, this process does not always induce the opening of stomata.
Malic acid in food
Malic acid contributes to the sourness of green apples. Malic acid is present in grapes. It confers a tart taste to wine, although the amount decreases with increasing fruit ripeness. The process of malolactic fermentation converts malic acid to much milder lactic acid.
Malic acid, when added to food products, is denoted by E number E296. Malic acid is the source of extreme tartness in USA produced confectionary; the so-called "extreme candy". It is also used with or in place of the less sour citric acid in sour sweets and Salt & Vinegar flavor potato chips. These sweets are sometimes labeled with a warning that excessive consumption can cause irritation of the mouth.
Chemistry
Malic acid was important in the discovery of the Walden inversion and the Walden cycle in which (-)-malic acid first is converted into (+)-chlorosuccinic acid by action of phosphorus pentachloride. Wet silver oxide then converts the chlorine compound to (+)-malic acid which then reacts with PCl5 to the (-)-chlorosuccinic acid. The cycle is completed when silver oxide takes this compound back to (-)-malic acid.
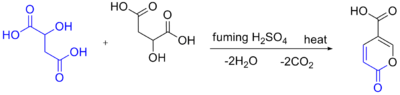
Self-condensation of malic acid with fuming sulfuric acid gives the pyrone coumalic acid:[3]
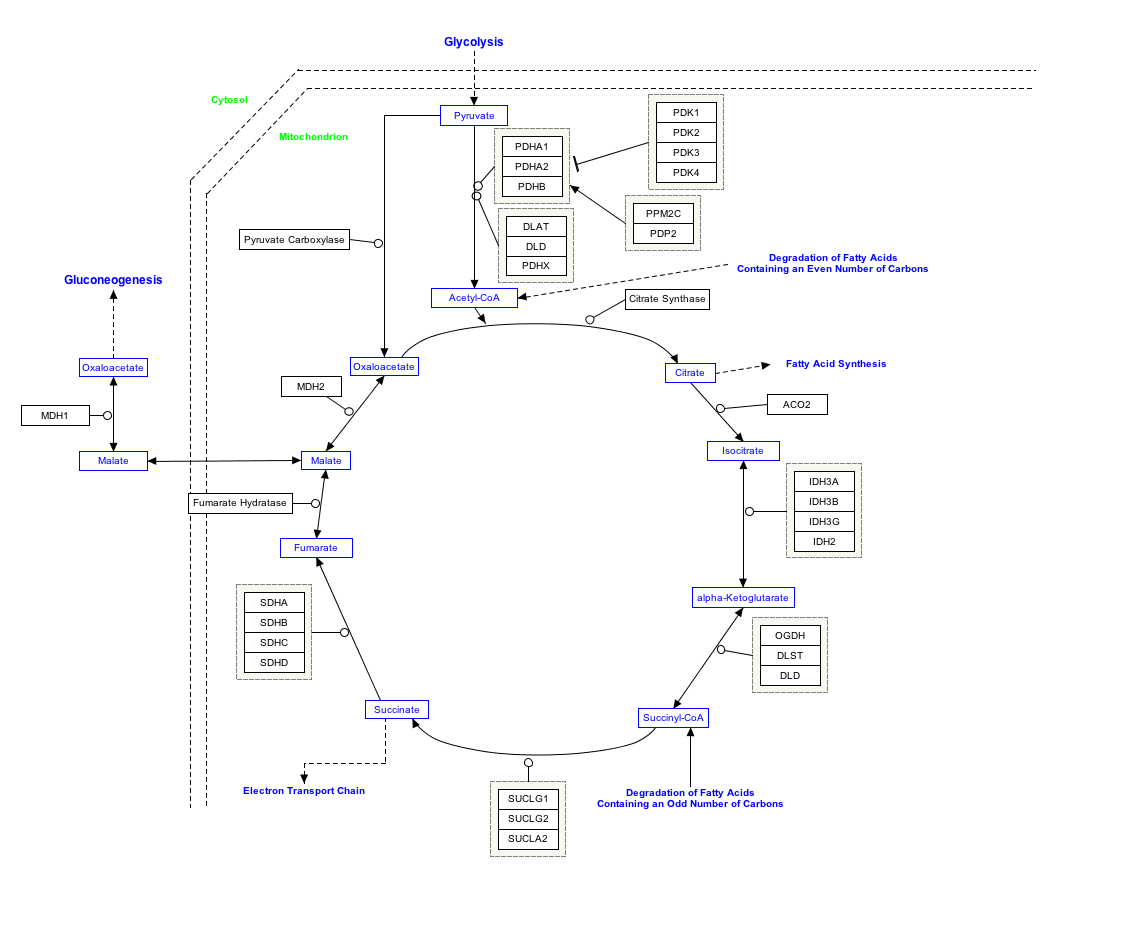
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [4]
See also
- Acids in wine
- Crassulacean acid metabolism
- Malate-aspartate shuttle
- Two other dicarboxylic acids have similar names and should not be confused with malic acid:
- Maleic acid
- Malonic acid
External links
References
- ↑ chemBlink Online Database of Chemicals from Around the World
- ↑ The Origin of the Names Malic, Maleic, and Malonic Acid Jensen, William B. J. Chem. Educ. 2007, 84, 924. Abstract
- ↑ Richard H. Wiley and Newton R. Smith (1963), "Coumalic acid", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0201; Coll. Vol. 4: 201
- ↑ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78". http://www.wikipathways.org/index.php/Pathway:WP78.
| Oxaloacetate | Malate | Fumarate | Succinate | Succinyl-CoA | ||||||||||||
| Acetyl-CoA | NADH + H+ | NAD+ | H2O | FADH2 | FAD | CoA + ATP(GTP) | Pi + ADP(GDP) | |||||||||
| + | H2O | NADH + H+ + CO2 | ||||||||||||||
| CoA | NAD+ | |||||||||||||||
| H2O | H2O | NAD(P)+ | NAD(P)H + H+ | CO2 | ||||||||||||
| Citrate | cis-Aconitate | Isocitrate | Oxalosuccinate | α-Ketoglutarate | ||||||||||||